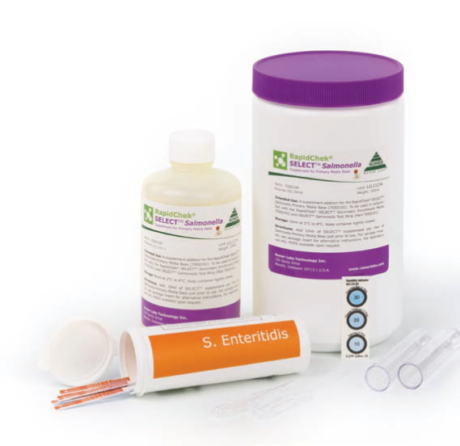
Product Description
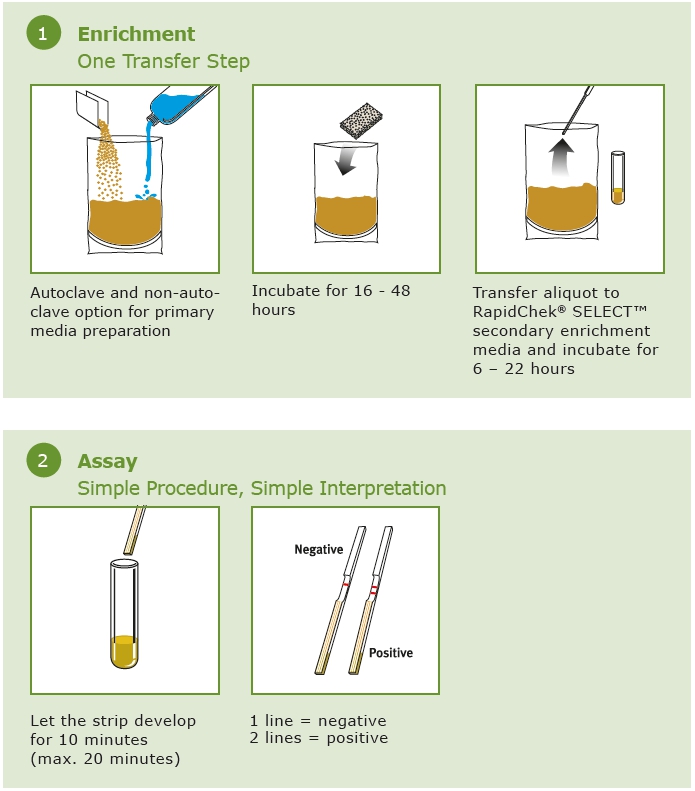
How the Test Works
The method uses a novel application of bacteriophages (or phage) in the media to act as selective agents, enhancing both the specificity and sensitivity of the overall method. Phages are bacterial viruses that attack cross-reactive bacteria. This prevents them from causing a false positive reaction (specificity). The phage also attack competitive bacteria, allowing for a media formulation that creates optimum conditions for rapid Salmonella Enteritidis growth (sensitivity). This patented media system is used in combination with a next generation RapidChek® SELECT™ Salmonella Enteritidis test strip. It contains proprietary anti-Salmonella D1 serogroup antibodies engineered to enhance the overall performance of the method. After 10 minutes, if Salmonella Enteritidis is present in the sample, two red lines will form. One line indicates a negative result. A control line is built into the test strip to indicate that the test has worked correctly. The test kits are stored at room temperature.
Applications
RapidChek® SELECT™ Salmonella Enteritidis has been designed to detect the pathogen in drag swabs, egg pools, and chicken carcass rinses.
Validations
The method is AOAC approved and awarded FDA equivalency.
Confirmation
Presumptive positive results must be confirmed by a cultural reference method (FDA BAM or USDA MLG). Drag swabs must follow the RapidChek® CONFIRM Salmonella Enteritidis IMS procedure prior to cultural confirmation. Egg pool samples must be confirmed by FDA BAM and chicken carcass rinses must be confirmed by the USDA MLG. At least two different types of selective agars should be plated for best results. RapidChek® Select Salmonella secondary media samples used in the test procedure can be used for confirmation.
Fast & Simple Procedure
- Fast product release
- Simplified media preparation
- Minimal training
- No additional equipment
Easy Resource Management
- High scalability
- Kit storage at room temperature
- Long shelf life
- AOAC approved
- FDA equivalency
Read the Package Insert instructions completely before performing any test.